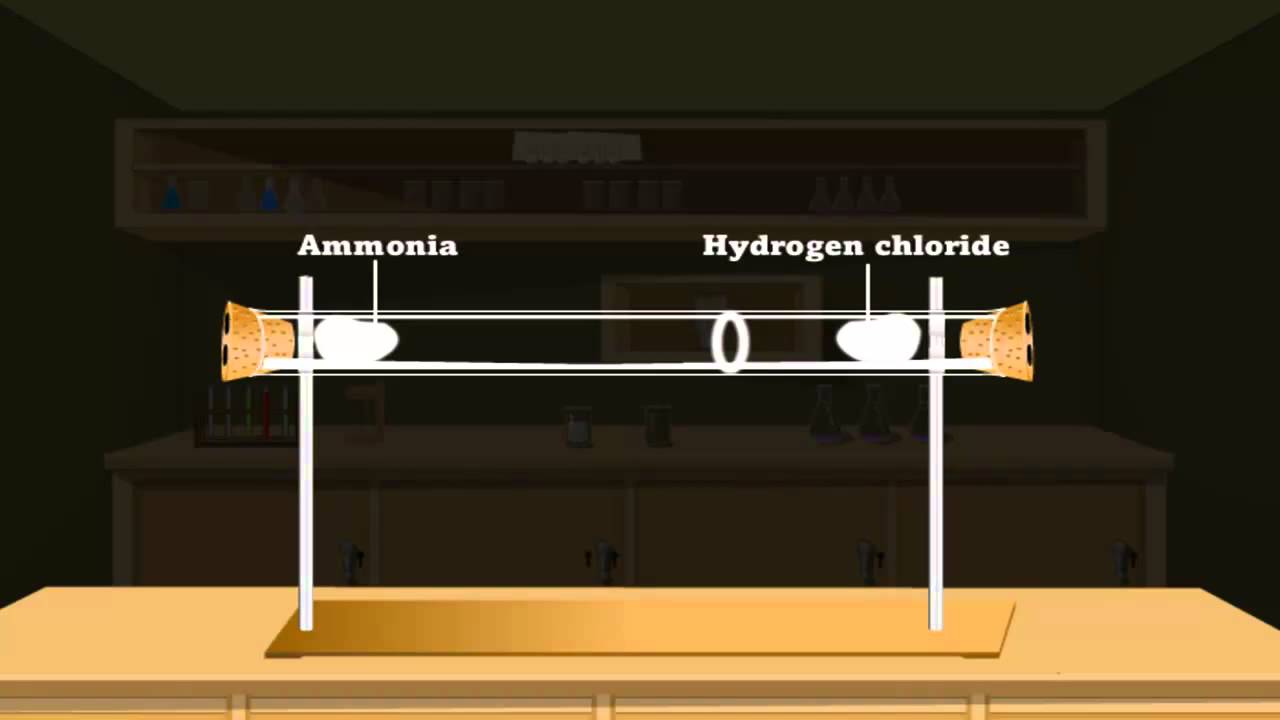
Diffusion Of Gases Lab Answers . How many times faster will. graham’s law of diffusion is based on the above assumptions of kinetic molecular theory. How to calculate the velocity of. how to perform a proper gas diffusion; diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The concentration gradient (the increase or decrease in concentration. both diffusion and effusion are related to the speed at which various gas molecules move. The theory can be stated in the. A balloon filled with helium gas. a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. effusion and diffusion of gases practice questions. Chemistry end of chapter exercises. Includes kit list and safety instructions. How to calculate theoretical and experimental average, and % error; Gases that have a lower molar mass.
from www.youtube.com
diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Chemistry end of chapter exercises. a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. How many times faster will. effusion and diffusion of gases practice questions. both diffusion and effusion are related to the speed at which various gas molecules move. The theory can be stated in the. the diffusion rate depends on several factors: graham’s law of diffusion is based on the above assumptions of kinetic molecular theory. how to perform a proper gas diffusion;
Diffusion in gases YouTube
Diffusion Of Gases Lab Answers The concentration gradient (the increase or decrease in concentration. both diffusion and effusion are related to the speed at which various gas molecules move. How to calculate the velocity of. How to calculate theoretical and experimental average, and % error; effusion and diffusion of gases practice questions. how to perform a proper gas diffusion; a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. How many times faster will. The concentration gradient (the increase or decrease in concentration. A balloon filled with helium gas. Chemistry end of chapter exercises. the diffusion rate depends on several factors: The theory can be stated in the. Includes kit list and safety instructions. graham’s law of diffusion is based on the above assumptions of kinetic molecular theory.
From www.slideshare.net
Diffusion of gases Diffusion Of Gases Lab Answers graham’s law of diffusion is based on the above assumptions of kinetic molecular theory. The concentration gradient (the increase or decrease in concentration. how to perform a proper gas diffusion; Gases that have a lower molar mass. effusion and diffusion of gases practice questions. A balloon filled with helium gas. both diffusion and effusion are related. Diffusion Of Gases Lab Answers.
From www.slideshare.net
Diffusion of gases Diffusion Of Gases Lab Answers a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. Includes kit list and safety instructions. How many times faster will. graham’s law of diffusion is based on the above assumptions of kinetic molecular theory. Gases that have a lower molar mass. effusion and diffusion of gases practice questions. How to calculate the. Diffusion Of Gases Lab Answers.
From diffuserjaika.blogspot.com
Answers To The Diffusion Througha Membrane Lab Diffusion Of Gases Lab Answers How to calculate theoretical and experimental average, and % error; Chemistry end of chapter exercises. The concentration gradient (the increase or decrease in concentration. both diffusion and effusion are related to the speed at which various gas molecules move. How many times faster will. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy.. Diffusion Of Gases Lab Answers.
From studylib.net
Diffusion of gases ammonia and hydrogen chloride Diffusion Of Gases Lab Answers Includes kit list and safety instructions. Chemistry end of chapter exercises. the diffusion rate depends on several factors: How to calculate the velocity of. Gases that have a lower molar mass. a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. effusion and diffusion of gases practice questions. The concentration gradient (the increase. Diffusion Of Gases Lab Answers.
From www.labxchange.org
LabXchange Diffusion Of Gases Lab Answers diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. How to calculate the velocity of. graham’s law of diffusion is based on the above assumptions of kinetic molecular theory. The theory can be stated in the. how to perform a proper gas diffusion; Chemistry end of chapter exercises. Includes kit list and. Diffusion Of Gases Lab Answers.
From www.youtube.com
Diffusion of gases Chemistry for All The Fuse School YouTube Diffusion Of Gases Lab Answers effusion and diffusion of gases practice questions. The theory can be stated in the. a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. How many times faster will. Chemistry end of chapter exercises. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. The concentration gradient (the increase. Diffusion Of Gases Lab Answers.
From diffuserdian.blogspot.com
Arrange The Following Gases By Diffusion Rate Diffusion Of Gases Lab Answers Gases that have a lower molar mass. the diffusion rate depends on several factors: effusion and diffusion of gases practice questions. how to perform a proper gas diffusion; both diffusion and effusion are related to the speed at which various gas molecules move. How to calculate theoretical and experimental average, and % error; graham’s law. Diffusion Of Gases Lab Answers.
From www.myxxgirl.com
Graham S Law Diffusion Of Ammonia Gas And Hcl G Chemdemos My XXX Hot Girl Diffusion Of Gases Lab Answers a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. how to perform a proper gas diffusion; How to calculate the velocity of. How many times faster will. Chemistry end of chapter exercises. Gases that have a lower molar mass. effusion and diffusion of gases practice questions. both diffusion and effusion are. Diffusion Of Gases Lab Answers.
From www.numerade.com
SOLVED Effusion is A special case of diffusion where gases diffuse Diffusion Of Gases Lab Answers The concentration gradient (the increase or decrease in concentration. Includes kit list and safety instructions. a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. graham’s law of diffusion is based on the above assumptions of kinetic molecular theory.. Diffusion Of Gases Lab Answers.
From www.studocu.com
Diffusion of gases 1 Diffusion of gases Also gases can diffuse Diffusion Of Gases Lab Answers The concentration gradient (the increase or decrease in concentration. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. How to calculate theoretical and experimental average, and % error; a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. How many times faster will. How to calculate the velocity of.. Diffusion Of Gases Lab Answers.
From www.chegg.com
Solved Experiment Graham's Law Diffusion of GasesI need Diffusion Of Gases Lab Answers Includes kit list and safety instructions. Chemistry end of chapter exercises. Gases that have a lower molar mass. How many times faster will. The theory can be stated in the. graham’s law of diffusion is based on the above assumptions of kinetic molecular theory. The concentration gradient (the increase or decrease in concentration. effusion and diffusion of gases. Diffusion Of Gases Lab Answers.
From brainly.in
12 Two gas jars each contain a different gas. The gas jars are Diffusion Of Gases Lab Answers both diffusion and effusion are related to the speed at which various gas molecules move. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Includes kit list and safety instructions. How to calculate the velocity of. Chemistry end of chapter exercises. The theory can be stated in the. Gases that have a lower. Diffusion Of Gases Lab Answers.
From www.chegg.com
Solved Experiment Graham's Law Diffusion of GasesI need Diffusion Of Gases Lab Answers How many times faster will. effusion and diffusion of gases practice questions. both diffusion and effusion are related to the speed at which various gas molecules move. a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. How to calculate theoretical and experimental average, and % error; Gases that have a lower molar. Diffusion Of Gases Lab Answers.
From www.sciencephoto.com
Gas diffusion Stock Image C043/2682 Science Photo Library Diffusion Of Gases Lab Answers How to calculate theoretical and experimental average, and % error; a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. The concentration gradient (the increase or decrease in concentration. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. both diffusion and effusion are related to the speed at. Diffusion Of Gases Lab Answers.
From www.numerade.com
SOLVED Q 4. An experiment was set up to compare the rate (diffusion Diffusion Of Gases Lab Answers the diffusion rate depends on several factors: Gases that have a lower molar mass. Includes kit list and safety instructions. How to calculate the velocity of. How to calculate theoretical and experimental average, and % error; a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. A balloon filled with helium gas. How many. Diffusion Of Gases Lab Answers.
From www.chegg.com
Solved I need help with question 1 (1 and 2) and the last Diffusion Of Gases Lab Answers How to calculate theoretical and experimental average, and % error; graham’s law of diffusion is based on the above assumptions of kinetic molecular theory. Includes kit list and safety instructions. How many times faster will. the diffusion rate depends on several factors: Chemistry end of chapter exercises. diffusion is faster at higher temperatures because the gas molecules. Diffusion Of Gases Lab Answers.
From www.chegg.com
Solved EXPERIMENT 9 DIFFUSION OF GASES AND GRAHAM'S LAW Diffusion Of Gases Lab Answers Includes kit list and safety instructions. The theory can be stated in the. a demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Gases that have a lower molar mass. A balloon filled with helium gas. The concentration gradient (the. Diffusion Of Gases Lab Answers.
From imat.entermedschool.com
Diffusion of gases chemistry Practice Question Solving Diffusion Of Gases Lab Answers Gases that have a lower molar mass. the diffusion rate depends on several factors: effusion and diffusion of gases practice questions. how to perform a proper gas diffusion; How to calculate theoretical and experimental average, and % error; The theory can be stated in the. A balloon filled with helium gas. Includes kit list and safety instructions.. Diffusion Of Gases Lab Answers.